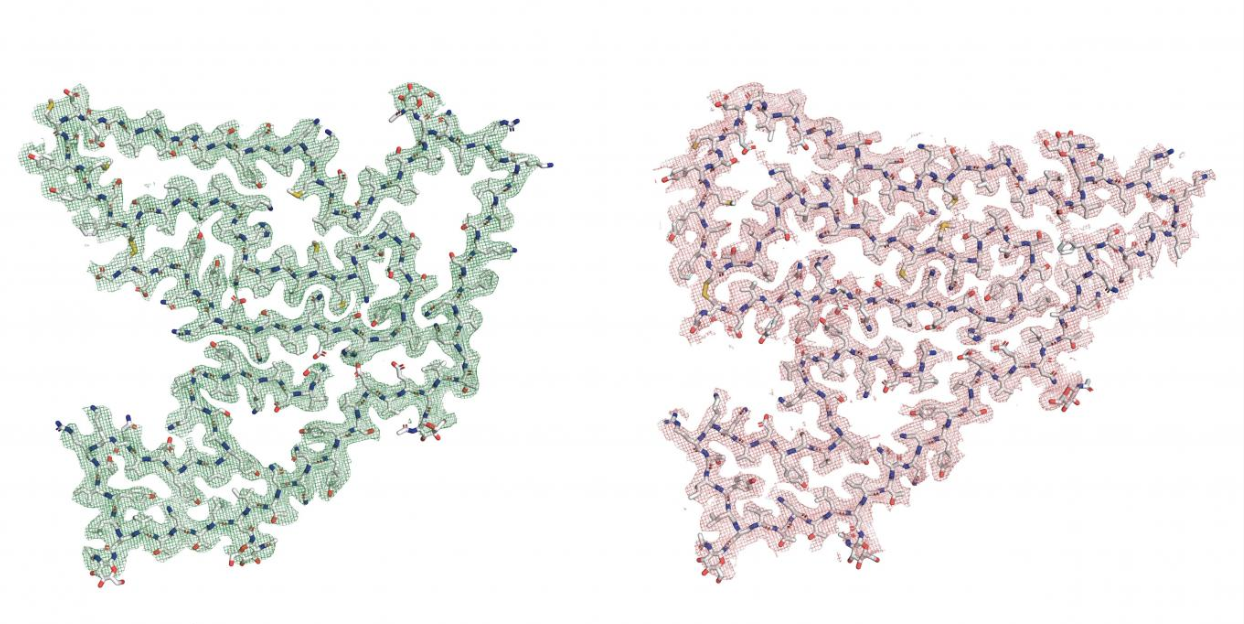A monumental new study published in Cell has revealed a startling and potentially game-changing discovery in neurodegenerative disease research. An international dream team of researchers found that a protein called TMEM106B, normally tasked with clearing out cellular junk, can abnormally clump into harmful aggregates known as fibrils. This bombshell finding provides the first evidence of a possible common mechanism underlying several detrimental brain disorders including Alzheimer’s disease, Parkinson’s disease, and frontotemporal dementia.
TMEM106B is a vital component of lysosomes and endosomes, which act as the waste disposal systems within cells, removing unwanted materials. The research team, comprising 24 collaborators across institutions worldwide, discovered that TMEM106B can split into fragments inside these organelles. The fragments then amass into fibrils, which are threads of misfolded proteins that are a hallmark pathology found in the brains of patients with neurodegenerative diseases.
To make this revelatory discovery, the researchers first extracted proteins from donated brain tissues of 11 deceased patients who had three distinct diseases associated with misfolded proteins. These diseases were progressive supranuclear palsy,multiple sclerosis, dementia with Lewy bodies, and frontotemporal lobar degeneration. The latter is the most common cause of dementia in those under 55 years old. Using cutting-edge cryogenic electron microscopy technology, the scientists took exquisitely detailed molecular snapshots of the brain proteins from multiple angles. This enabled them to generate intricate 3D models unveiling the proteins’ structures at an atomic level. Shockingly, the models matched a 135-amino acid fragment of TMEM106B rather than any of the known fibril-forming proteins like tau in Alzheimer’s.
A protein normally involved in clearing cells of molecular debris can clump into fibrils, potentially hobbling cells
The identification of TMEM106B is monumental because over a decade ago, genetics studies had already linked variants in this gene with increased risk of frontotemporal lobar degeneration. The new bombshell finding that TMEM106B fibrils are present across several neurodegenerative brain diseases points to a potentially causative role in disease pathogenesis.
The researchers speculate that the formation of toxic TMEM106B fibrils cripples the normal function of lysosomes and endosomes. This impairment triggers a buildup of other damaging protein aggregates that are the cardinal features of Alzheimer’s disease, Parkinson’s, and related neurodegenerative brain disorders. The accumulation of misfolded proteins is believed to ultimately destroy neurons, resulting in the symptoms of dementia, parkinsons disease movement abnormalities, and speech issues seen in these diseases.
Additional studies are still imperative to conclusively prove that TMEM106B fibrils directly induce neurodegeneration. However, this astronomical discovery sets researchers on an exciting new trajectory. It raises the tantalizing possibility that dysfunction of the lysosomal pathway could be the elusive common thread linking these varied neurodegenerative diseases that were previously thought to be unrelated. Untangling this biological convergence at last could pave the way for pioneering treatments to slow or arrest the progression of these currently incurable and devastating brain disorders that collectively afflict millions of patients worldwide.
The researchers emphasized that this major scientific coup was only achievable thanks to the selfless donation of brain tissue by deceased patients to further research. This highlights the immense value of brain and tissue donation in catapulting solutions to complex diseases forward at warp speed. In particular, the study spotlights the importance of including less mainstream neurodegenerative diseases like progressive supranuclear palsy and frontotemporal dementia, in addition to more widely-known disorders such as Alzheimer’s.
Though more research is needed, the unexpected identification of TMEM106B fundamentally transforms the field and rekindles hope for unraveling the mysteries of neurodegenerative disease. After decades of failed clinical trials targeting amyloid and tau, proteins long considered the main culprits, researchers finally have an exciting new lead to pursue. The monumental discovery of TMEM106B’s role may finally interlace the intricate web linking these tragic and incurable conditions, illuminating the path towards prevention and treatment. For the millions of patients and families devastated by the ravages of Alzheimer’s, Parkinson’s, and related dementias, this watershed breakthrough kindles cautious optimism in the long battle ahead.